
Solar Absorption at Your Command:
Bands vs. Lines, Broad vs. Narrow
How to Find Awesome Features in the Sun's Spectrum
The Standard Catalog of Henry
Rowland and Charlotte Moore
Background: In the late 1880s, Henry Rowland, working at The Johns Hopkins University in Baltimore with diamond-ruled, concave, diffraction gratings, recorded the first, high-resolution photographic atlas of the solar spectrum. "By comparing the observed solar lines with laboratory spectra, Rowland" was able to report "the presence of 39 chemical elements in the sun." In 1928, Charlotte Moore converted "the Rowland wavelength scaleů to the international scale" and published The Solar Spectrum 2935┼ to 8770┼: Second Revision of Rowland's Preliminary Table of Solar Spectrum Wavelengths. Miss Moore, then at the National Bureau of Standards, reported 57 elements and identified 57% of the sun's absorption lines. Moore, however, did not " attempt... to replace the estimated line intensities from Rowland's Table by measured equivalent widths." Equivalent widths first were catalogued in 1943 at the Utrecht Observatory in the Netherlands using plates taken at Mt. Wilson Observatory, a project that continued after the War under the auspices of the International Astronomical Union. The last, hard-copy edition of Charlotte Moore's The Solar Spectrum was published in 1966 and reported 63 elements, identified 73% of all absorption lines, and gave equivalent widths for 97% of them. Now a standard reference, it is available today over the Internet at ftp://ftp.noao.edu/fts/linelist/Moore. Nearly every solar absorption line in the interval 3850┼ to 6900┼, including many very faint absorption lines, can be observed with Questar's
TM.
Concepts: The intensity of an absorption line is measured by its equivalent width,
. The equivalent width is the width that a pure black line would have to have in order to enclose the
same area underneath the 100% emission level, known as the continuum, that the actual line encloses. Note that there is always some residual emission in the center of an absorption line. Even the most intense and broadest lines, such as Ha, which, through the
TM "looks like a tree trunk", are never entirely black. Absorption lines also have wings, which give to each line its shape. The wings tell us what interactive, atomic and/or subatomic processes are at work to broaden the absorption. High-temperature, or Doppler, broadening is apparent in many solar lines. Natural broadening, a direct consequence of the Heisenberg Uncertainty principal affecting atoms deep in the sun's atmosphere, is far more difficult to observe. With the
's extraordinary resolution, however, natural broading can in fact be seen: in the feathery, wispy wings - which look out of focus but aren't! - of the magnesium lines at 5183┼ and 5173┼.
Columns and Symbols: The Rowland/Moore catalog at ftp://ftp.noao.edu/fts/linelist/Moore presents data in seven columns for each spectral line and uses a variety of symbols
Column 1 provides the wavelength in Angstrom units (┼) for each absorption line. The
TM wavelength dial reads directly in nanometers (nm) in intervals of 0.2nm (= 2┼). A wavelength followed by the letter S is a standard wavelength. Lines first measured by Charlotte Moore on Mt. Wilson plates are followed by the letter M. Lines first measured by Henry Rowland are followed by the letter R. Lines first measured at the Utrecht Observatory and published in its 1940 Atlas are followed by the letter A
Column 2 provides the equivalent width,
, of each absorption line in thousandths of an Angstrom unit. In the examples below, the prominent iron (Fe) line at 4045.825┼ has an equivalent width of 1.174┼.
Column 3 is really a double column. Before the period is the so-called reduced width,
, a dimensionless number expressed in units called Fraunhofers. Behind the period is the appearance of the line in sunspots, according to the following designations:
SS the line is greatly strengthened in sunspots
S the line is strengthened in sunspots
U the line is unchanged in sunspots
WW the line is greatly weakened in sunspots
W the line is weakened in sunspots
O the line is obliterated in sunspots
N the line is diffuse in sunspots
NNNN the line is very diffuse in sunspots
D the line is double in sunspots (an uncommon condition unrelated to the Zeeman effect)Column 4 identifies the element responsible for the absorption line followed by a space and then by a number to indicate the element's state of ionization. CA 1, for example, indicates Calcium in the ground state, also written CA I. Similarly, TI 2 indicates singly ionized Titanium, also written Ti II. Molecules are identified by their chemical formulae as, for example, by CN for cyanogen. When a number follows a letter with no intervening space - as, for example, C2 - then the number functions as a subscript in a molecular formula: thus C2 is C2. Unresolved blends of lines are indicated by // or by / or by - . Telluric lines, originating in the earth's atmosphere, are identified by ATM. Note that emission phenomena, such as the Helium emission line at 5876.7┼, are not listed in the Rowland/Moore catalog.
Column 5 provides a measure of the energy needed to bring the absorbing atom into the lower level of the transition that produces the absorption. This needed energy, called the low excitation potential, is measured electron volts, or eV. For a molecule, the entry in column 5 relates to the quantum-mechanical description of the molecule.
Column 6 identifies the so-called multiplet number of an atomic absorption line, or the vibration band for a molecular absorption line. Column 6 allows you to identify related absorption lines.
Column 7 indicates which numbered note to consult at the end of The Solar Spectrum for further information about the absorption. Sometimes it is possible to purchase a copy of The Solar Spectrum over the Internet for a modest price. Web sites to consider are http://www.addall.com and http://www.abebooks.com/.
If the lines constituting a blend have been separately identified, then they will be given individually, as in the example below, at 4276.680┼.
NOTE: The columns in the Internet version of The Solar Spectrum are not labeled, as they are here. The examples below, all from within the
TM wavelength range, will help you to find your way around.
Wavelength
Equivalent Width
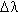
Reduced
Width.
Appearance
in SunspotElement or Molecule
Low Excitation Potential in eV
Multiplet Number or Vibration Band
Note
3850.165M
59.
27.
CN
R28
0,0
11
4045.825M
1174.
316.SS
FE 1
1.48
42
4091.999R
9.
2.S,NN
CA 1
2.93
42
4146.234A
18
4.S,D
CR 1
3.84
260
17
4276.680S
57.
13.U
//FE 1
3.27
597
4276.680S
57.
13.U
//FE 1
3.88
976
4276.680S
57.
13.U
TI 1
2.30
252
4320.958M
63.
16.W
TI 2
1.16
41
4340.475M
2855
659.WW,NN
H
10.20
1
5010.218
25.
5.0
TI 2
3.09
113
5088.543
26.
5.S,NNNN
NI 1-
3.85
190
5094.026
9.
2.U"
C2-
P62
0,0
17,19,57
5891.660
18.
3.
ATM H20
R3
302
26
6900.543M
4.
1.
ATM 02
P6
1,0
23